
Tree of life
by Nickoli Parkinson & Editor Elizabeth Lugones
Before discussing cellular senescence, we must first discuss the problem, aging. As we know, during the aging process, organisms undergo significant physiological changes. And it is these changes that sometimes/inevitably lead to the development of chronic diseases such as cancer, neuro-degeneration and many others. Thus, aging can be identified as a key marker in the development of these conditions.
As it stands, billions of dollars have been invested in age-related research in the attempt to develop treatments to these conditions, and this has contributed to many discoveries. However, there remains a large gap between where we are now and developing functional cures for these conditions .
Recently however, arose a new line of thinking among many scientist around the world. If aging is a major cause of the development of these conditions, why not eradicate them by preventing aging all together! And this is exactly what the research suggest. Research suggests that the development of these chronic diseases can be prevented if the aging process is subverted. But before we discuss this let us discuss what aging is.
What is Aging?
To understand how the onset of age-related diseases can be prevented by blocking the process of aging, we first must understand what aging is. Aging can be defined as the progressive loss of molecular fidelity after reaching sexual maturity. In other words, there is a decrease in the efficacy of molecular machinery in an organism.
These molecular machineries, known more commonly as a biochemical pathways, are responsible for maintaining homeostasis and ensuring the continued survival of the organism throughout its lifespan (not to be confused with helathspan, two distinct terminologies). However, once reproductive maturity is reached, the efficacy of these molecular machinery gradually deteriorates.
As a result when mammals age, they become more susceptible to diseases like cancer, cardiovascular conditions, and neurodegeneration. These often times can be attributed to the before mentioned decrease in molecular fidelity.
What is Cellular Senescence?
To be clear, the study of these various models has uncovered a variety of different factors considered to contribute to the aging process. Genetic differences, somatic mutations, and non-genetic factors, known collectively as epigenetics, have all been discovered to underly the processes of aging. But in this article, we focus specifically on Cellular senescence.
Cellular senescence is described as “a state of permanent cell cycle arrest” in cells due to various stressors induced on the cell, has recently been highlighted as a prime suspect in the process of aging and age-related diseases. Thus, making it a prime target for research and therapeutic exploitation. The past half century has been very crucial in amassing information on senescence. But this has been largely confined to senescence in vitro. Senescence in vivo, or in living organisms, still largely remain unstudied.
A Deeper Look at Cellular Senescence
In forcing a permanent cell cycle arrest, senescence prevents the propagation of damaged or cancerous cells. Cellular senescence also forces cells to undergo other changes as well. Metabolic reprogramming, such as the activation of the INK4/ARF locus, which is normally silent in regular cells, chromatin rearrangement, such as the construction of senescence associated heterochromatic foci (SAHFs), and autophagy modulating, stemming for the creation of SAHFs, all are phenotypic alterations undergone by senescent cells. It was after observing that aged tissue contains many senescent cells, the connection between senescent cells and aging was first alluded to.
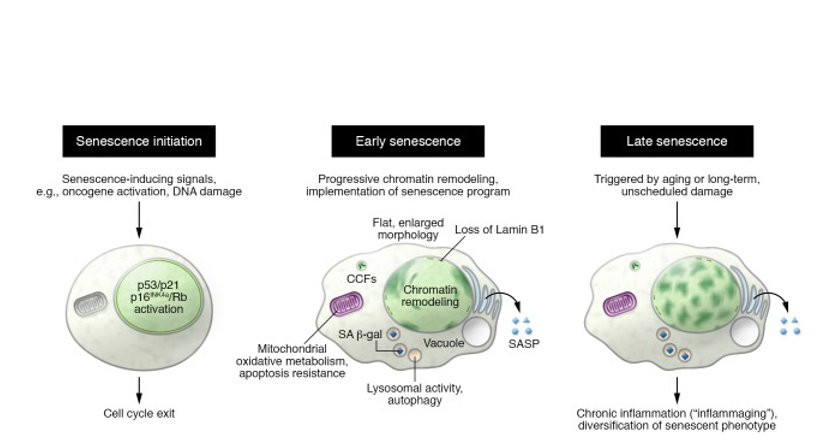
Figure 1. From “Mechanisms and functions of cellular senescence” by Nicolas Herranz and Jesus Gil. This diagram depicts some phenotypic alterations between different stages of senescence
Senescence induces cells to exit the cell cycle via the activation of p53/p21CIP1 and p16INK4a/Rb tumor suppressor pathways. Known as “the guardian of the genome”, p53 functions normally as a tumor suppressor. In senescence, p53 induces transcript of p21CIP1 a cyclin dependent kinase inhibitor (CDKi), whose function is then to block CDK2 activity. This results in a hypo phosphorylation of Rb and subsequent cell cycle exit. In stresses that are transient, p53 can also activate DNA repair processes which in turn leads to cellular senescence.
Transient stresses can also activate p16INK4a, which inhibits CDK4 and CDK6. This contributes to a continuing arrest of the cycle. Different stressors, such as DNA damage, can also induce senescence-associated arrest of the cell cycle. If this DNA damage is persistent, this can lead to irreparable DNA damage causing senescence.
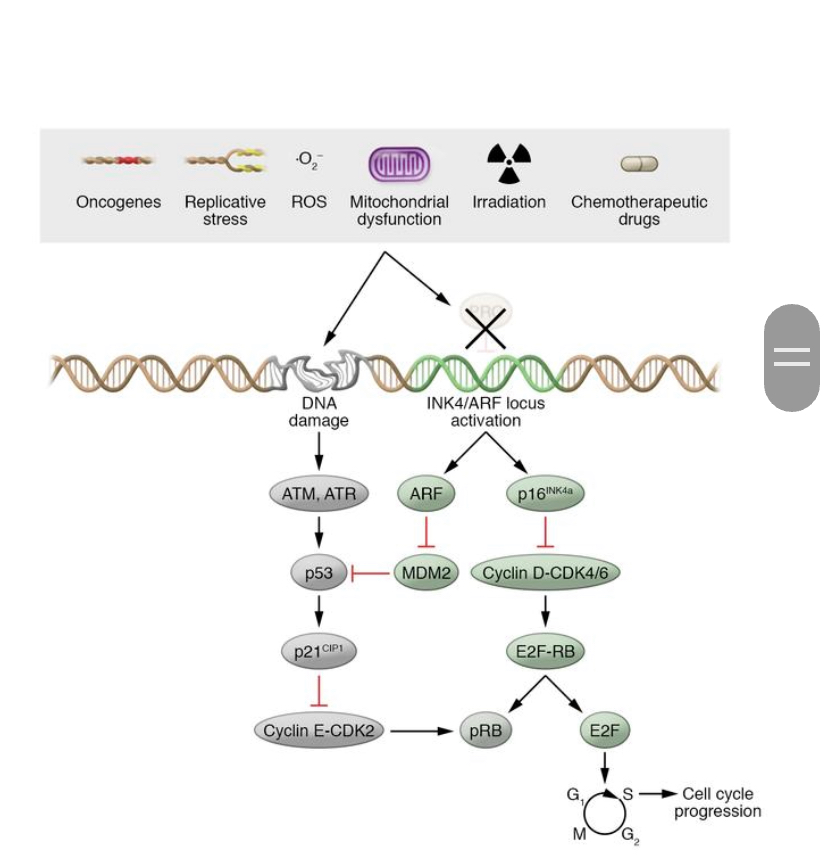
Figure 2. From “Mechanisms and functions of cellular senescence” by Nicolas Herranz and Jesus Gil. Many different types of stressors induce senescence-associated growth arrest. p53/p21CIP1 and p16INK4a/Rb pathways regulates the cell cycle.
A group of genes, that are responsible for regulating senescence, were identified as drivers of aging. Typically, senescence plays an important role in the uncontrolled proliferation of cells, i.e., cancer. But we also see derivative effects, in which tissues repair facilities in progenitor cells are lost due to cell cycle arrest and proinflammatory and matrix-degrading molecules are produced as a result of senescence-associated secretory phenotypes, otherwise known as SASP. Typically, these SASP have a myriad of functions but a major function highlight of SASPs is that SASPs can mobilize immune response due to differing pathogeneses. Though there are many layers of SASP regulation, nuclear factor κB (NF-κB) and CCAAT/enhancer–binding protein beta has been shown to be key regulators for SASP. As a result of these discoveries, destroying senescent cells in the body and SASP therapies are both being considered as potential antagonisms of the ageing process.
Cellular Senescence and Aging
Aging is considered to be a pleiotropic process that occurs gradually in which tissue is lost and organs fail over time1. Currently, the theory is that aging is a result of genetic mechanisms that favor reproductive fitness early in life but does not emphasize this same fitness later in life.1 To understand how senescence fits into aging, first you have to understand hallmarks of aging. They are categorized as primary, antagonistic, and integrative.
The category primary refers to the causes associated with age-associated damage, antagonistic refers to the subsequent responses to that damage, and integrative refers to the overall phenotype of the previous two categories. Senescence is considered an antagonist since senescence is a response to events considered to be in the category of primary when considering aging. It should be noted however that senescence has also been shown to influence the integrative category of aging as well.
Now that we’ve covered broadly what cellular senescence is and its role, lets focus exactly on how senescent cells contribute to the aging process. As we age, there is increased chances for the occurrence of cancer. Though senescence plays an unequivocally important role in preventing cancer, there is evidence that the presence of a large number of senescent cells in aged tissue actually provide fertile ground for cancerous cells to thrive (Krtolica 2001). Mentioned before is the SASPs stemming from the senescent cells. Suggested in the literature is that the accumulation of the products of the SASPs can actually have deleterious in the surrounding environment of these cells by creating setting that promotes the formation of cancerous cells.
Cardiovascular disease is also another disease whose occurrence increases with the advancement of an organism’s age. Confirmed is that senescence has a role in the development of atherosclerosis, a cardiovascular disease caused by plaque deposition (Childs 2016). It is unclear however what role exactly senescence cells play in the development of atherosclerosis, but it was shown in experimental models that the removal of these senescent cells had a beneficial effect of the development of this disease.
Another major deleterious development in the aging process is the decline in the function of an organism’s immune system fidelity. As organisms age the less efficient, they become in rising an immune response due to the decline in function of Hematopoietic Stem Cells (HSCs). This is due to a rise in senescent HSCs. It has been shown that removal of the senescent HSCs and replacing them with normal cells rejuvenates the immune system (Chang 2015).
Sarcopenia is generally defined as the loss of muscle tissue due to the process of aging. As we age muscle tissue and cells lose their ability to repair and replenish themselves. Normally quiescent, what we see is that these cells become senescent with time. The removal of these senescent muscle cells can rejuvenate the ability of the remaining cells to regain their fidelity.
These are just a few of the myriad of diseases that senescence contributes to. Senescence also contribute to osteoarthritis, fatty liver disease, IPF, type 2 diabetes and renal dysfunction. Though the role that senescent cells may have in the development of these age-related conditions may sometimes be unclear and elusive, a clear correlation in the development of these disease and the presence of senescent cells have been established in the lab.
Eliminating senescent cells as therapy for aging
Due to the many pleiotropic nature of senescence in a variety of age-related diseases, researchers have suspected and demonstrated that removing or preventing senescence cells from developing is a viable pathway for delaying the onset of the overall health decline in an organism attributed to aging. Previously, inhibiting the P53 and p16 pathways or lengthening telomeres were examined as a targeted therapy for prevented the age-related decline of our health but both of these pathways in turn increased the occurrence of cancer in an individual. Removing senescent cells has shown to be a far safer alternative than these other therapies.
Senescent cells rely on antiapoptotic pathways to maintain their “lifespan” in tissues. These pathways include the Bcl-2/Bcl-xL pathway which helps regulate apoptosis induced by mitochondria. A class of drugs known as “Senolytics”, drugs that selectively induce death in senescent cells, have been discovered. These include dasatinib, which disrupts the EFNB receptor, which upon binding of the signal molecule EFN B3 prevents apoptosis, and quercetin, which inhibits ACT/Pe3k, a protein in the Bcl-2/Bcl-xL apoptosis pathway. These discoveries have stimulated research into these class of drugs. Currently with these drugs having been identified and though the first clinical trials have yet to be started, preclinical models have been developed. In these models, Senolytics delay and prevent over 40 different conditions. Early pilot trials have shown Senolytics to reduce frailty in humas (Kirkland 2020). However, due to the already ongoing studies in COID-19, Alzheimer’s disease, osteoarthritis and osteoporosis, Senolytics cannot yet be used in clinical trials.
Closing Statement
It also should be noted that the specific incidence to which senescence plays a role in many of these diseases have not yet been established, however it should also be noted that, despite this seemingly red flag, it has been proven that there exists a clear correlation between these two variables.
A key feature in aging is the decline in tissue regenerative capabilities. This decline results in loss of function of the correlating organ and can be traced back to the stem cells of these specific organs. Restoring the regenerative capabilities of these cells by inducing the removal of these cells from a state of senescence, or removing the cells entirely, seems to be a safe and viable pathway to combat the detrimental process of aging. Not touched upon in the paper was the potential detrimental effects of the interference of these senescent cells, but previous studies have shown that restoring the proliferative mechanism in senescent sells can cause teratomas to form in the organs which received such treatment. This suggest that elimination of these cells, not reverting to a proliferative state, is the more viable therapy to combat the age-related illnesses caused by senescence.
Citations
Childs, B., Baker, D., Wijshake, T., Conover, C., Campisi, J., & Deursen, J. (2016, October 28). Senescent intimal foam cells are deleterious at all stages of atherosclerosis. Retrieved December 12, 2020, from https://science.sciencemag.org/content/354/6311/472
Childs, B., Durik, M., Baker, D., & Van Deursen, J. (2015, December). Cellular senescence in aging and age-related disease: From mechanisms to therapy. Retrieved December 12, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4748967/
Coppé, J. (n.d.). The Senescence-Associated Secretory Phenotype: The Dark Side of Tumor Suppression. Retrieved December 12, 2020, from https://www.annualreviews.org/doi/full/10.1146/annurev-pathol-121808-102144
Herranz, N., & Gil, J. (2018, April 2). Mechanisms and functions of cellular senescence. Retrieved December 12, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5873888/
Kirkland, J., & Tchkonia, T. (2020, November). Senolytic drugs: From discovery to translation. Retrieved December 12, 2020, from https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7405395/
Krtolica, A., Parrinello, S., Lockett, S., Desprez, P., & Campisi, J. (2001, October 09). Senescent fibroblasts promote epithelial cell growth and tumorigenesis: A link between cancer and aging. Retrieved December 12, 2020, from https://www.pnas.org/content/98/21/12072.short
Soto-Gamez, A., & Demaria, M. (2017, January 19). Therapeutic interventions for aging: The case of cellular senescence. Retrieved December 12, 2020, from https://www.sciencedirect.com/science/article/pii/S135964461730017X




More Stories
Using CRISPR Chinese Scientists Successfully Slow Down Aging in Mice